Lifestyle
Charles Winn gives the Lowdown on the World’s ‘Fine Wine’ Capital
The majority of the world might remain in lockdown amid the ongoing coronavirus pandemic, but for some industries life must continue as normal, such as for ‘fine wine’. Whilst the rest of the world locks down, in Bordeaux – the fine wine capital of the world – producers are hard at work preparing their crops for the next season.
Geography
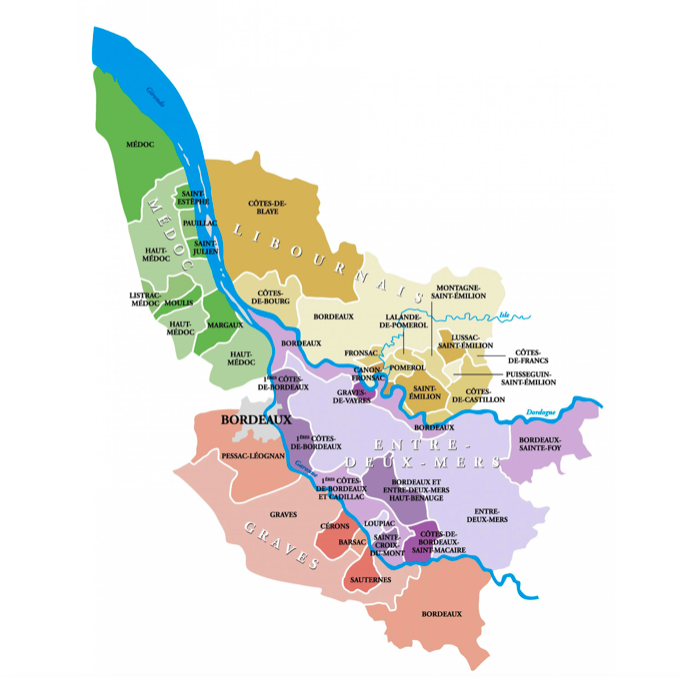
A port city on the Garonne River in southwestern France, Bordeaux is renowned globally for its famous wine-growing regions. A river runs directly through the region, and on the West side sits Gironde and Garonne. Typically, these regions are known for wines such as Sauvignon. On the East side of the river nestles Dordogne, known primarily for Merlot.
In total, Bordeaux has 57 grape-growing regions making it the biggest wine producer in France. Originally made famous for its popularity with kings, nowadays, Bordeaux and its chateaus are popular tourist attractions.
The Wines
As one of the biggest wine-makers in the world, you might expect Bordeaux to produce a diverse range of different type of wines. However, more than 90% of the wine produced here is actually red, with the region specifically producing Cabernet Sauvignon, Cabernet Franc, Merlot, Petit Verdot, Malbec and Carménère.
Having said this, in 2019 Bordeaux’s regulatory body approved four additional dark grape varieties to add to the list: Marselan, Touriga Nacional, Castets, and Arinarnoa.
Bordeaux’s First Growth wines (the term for a wine made specifically made in Bordeaux) are made by blending 70% Cabernet Sauvignon, 15% Cabernet Franc and 15% Merlot. In contrast, the White Bordeaux is made from Sémillon, Sauvignon Blanc and Muscadelle.

The History
Bordeaux’s wine-making history stretches back over many centuries. In 1855, the Association of Bordeaux Wine Merchants established official classification and certification of the wines after Emperor Napoleon III requested that they do so.
Ranking the wines from First Growths to Fifth Growths, the merchants evaluated market prices based on an evaluation of the previous years. They noticed that red wines which made the list came from the Médoc region, except for one: Château Haut-Brion from Graves.
Since this original classification in 1855, there have only been two changes. In 1856, Château Cantemerle was added as a Fifth Growth and in 1973, Château Mouton Rothschild was promoted from Second Growth to the elite First Growth classification. The latter change is a wine much loved and highly ranked by Charles Winn and its customers.

Château Margaux
Global flavour
It didn’t take long for the popularity of Bordeaux wine to grow all over the world. After King Henry II’s marriage to Eleanor of Aquitaine in 1152, an interest in wine from the Bordeaux region was prompted in England.
The marriage established the province of Aquitaine within France and England, and a new dark rosé wine was created, called ‘Claret’. This wine soon became the most common wine to be exported to Britain.
After the battle of Castillion in 1453, the Aquitaine region returned to the French. Since then, the word ‘Claret’ became anglicised and is still widely used today, due to the global popularity of the wine.

The exterior of the château
Lifestyle
When Seasons Shift: Dr. Leeshe Grimes on Grief, Loneliness, and Finding Light Again

Some emotional storms arrive without warning. A sudden change in weather, a holiday approaching, or even a bright sunny day can stir feelings that don’t match the world outside. For many people, the hardest seasons are not defined by temperature; they are defined by what’s happening inside, where grief and loneliness often move quietly.
This is the emotional terrain where Dr. Leeshe Grimes has spent her career doing some of her most meaningful work. As a psychotherapist, registered play therapist, retired U.S. Army combat veteran, and founder of Elevated Minds in the DMV area, she understands how deeply seasonal shifts and unresolved grief can affect people. Her upcoming books explore this very space, guiding readers through the emotional weight that can appear during different times of the year.
What sets Dr. Grimes apart is her ability to see clearly what many people overlook. Seasonal depression, for example, is usually tied to winter months. But she often sees it appear during warm, bright seasons, the times when the world seems happiest. For someone already grieving or feeling disconnected, watching others travel, celebrate, or gather can create its own kind of heaviness. Sunshine doesn’t always lift the mood; sometimes it highlights what feels missing.
The same misunderstanding surrounds grief. Society often treats it as a short-term experience with predictable phases and a clean ending. But in her practice, Dr. Grimes sees how grief keeps evolving. It doesn’t disappear on a timeline. It weaves itself into routines, memories, and milestones. People learn to carry it differently, but they rarely leave it behind completely. And that’s not failure, it’s human.
Her approach to mental health centers on truth rather than pressure. She encourages clients to acknowledge the emotions they try to hide: sadness that lingers longer than expected, moments of joy that feel out of place, and the waves of loneliness that return even when life seems stable. Instead of pushing for quick recovery, she focuses on helping people understand how emotions shift and how to care for themselves through those changes.
Much of her insight comes from her military years, where she witnessed the emotional toll of loss, transition, and constant survival. She saw how people continued functioning while carrying pain that had nowhere to go. That experience shaped her belief that healing requires space, space to feel, to speak, and to move through emotions without judgment.
In her clinical work today at Elevated Minds, she encourages people to build small, steady habits that anchor them during difficult seasons. Journaling helps them recognize patterns and name what feels heavy. Community support breaks the cycle of isolation. Therapy creates a place where emotions don’t have to be minimized or explained away. And intentional routines, daily sunlight, mindful breaks, and calm evenings help rebuild emotional balance.
Her upcoming books expand on these ideas, offering practical guidance for navigating both grief and seasonal depression. She focuses on helping readers understand that healing is not about escaping pain. It’s about learning how to live with it in a healthier way, honoring memories, acknowledging loneliness, and still allowing room for moments of light.
What makes Dr. Leeshe Grimes a compelling voice in mental health is her ability to bring language to experiences that many struggle to explain. She reminds people that emotional seasons don’t always match the weather and that there is no single path through grief. But within those shifts, she believes there is always a way forward.
The seasons will continue to change. And with the right tools, compassion, and support, people can change with them, finding steadiness, softness, and light again, one step at a time.
-

 Tech5 years ago
Tech5 years agoEffuel Reviews (2021) – Effuel ECO OBD2 Saves Fuel, and Reduce Gas Cost? Effuel Customer Reviews
-

 Tech6 years ago
Tech6 years agoBosch Power Tools India Launches ‘Cordless Matlab Bosch’ Campaign to Demonstrate the Power of Cordless
-

 Lifestyle7 years ago
Lifestyle7 years agoCatholic Cases App brings Church’s Moral Teachings to Androids and iPhones
-

 Lifestyle5 years ago
Lifestyle5 years agoEast Side Hype x Billionaire Boys Club. Hottest New Streetwear Releases in Utah.
-
 Tech7 years ago
Tech7 years agoCloud Buyers & Investors to Profit in the Future
-

 Lifestyle5 years ago
Lifestyle5 years agoThe Midas of Cosmetic Dermatology: Dr. Simon Ourian
-

 Health7 years ago
Health7 years agoCBDistillery Review: Is it a scam?
-

 Entertainment7 years ago
Entertainment7 years agoAvengers Endgame now Available on 123Movies for Download & Streaming for Free
